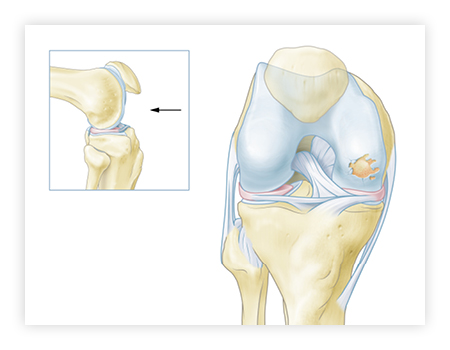
In the EU and US alone, there are several million patients per year diagnosed with Traumatic Focal Defects (TFD) in the cartilage of the knee. In addition to the knee, TFDs in hip and small joints is estimated to represent in US and EU over 1 million patients per year.
These defects often result from sports injuries and falls. During a fall the articular cartilage can become damaged as a result of blunt trauma. This can be caused by a forceful strike from a kicking leg or even a fast-moving cricket ball, but also resulting from a wrong move, during sports, dancing or simply walking.
 |
 |
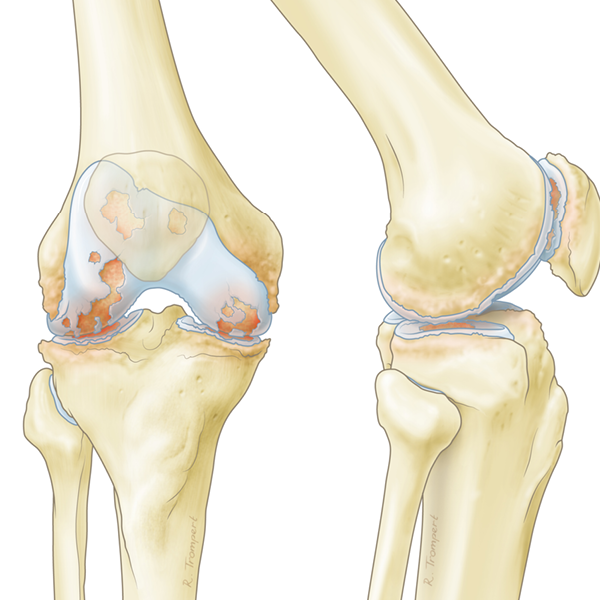
Osteoarthritis (OA) is a long-term chronic disease characterized by the deterioration of cartilage in joints triggered by trauma and/or wear and tear of the joints. OA is one of the most devastating chronic conditions that affects people around the world. Although the usual population associated with the condition are the elderly, who are mostly inactive, also athletes and younger individuals are susceptible. While in the elderly wear and tear is often the cause for OA, sports injuries and falls are frequently causing OA in the younger patient population.
Cartilage is the soft tissue on the distal ends of bones in our joints. It acts as a shock absorber and is also very slippery, facilitating the smooth and pain-free sliding of the bones alongside each other.
Compared with other body tissues, cartilage has no blood supply, no nerve innervation, and cells are adhered in a matrix, which restricts the potential to heal when injured.
An important factor for the long-term success of a cartilage repair intervention is the type of cartilage tissue that is formed during healing. Most techniques result in the formation of scar tissue. This fills the defect and provides temporal relief of complaints. Scar tissue is, however, inferior with respect to shock absorption and resistance to wear and tear of the moving joint. For durable cartilage repair the defect needs to be filled with so-called hyaline cartilage. This type of tissue is evolved during evolution to meet the specific demands for pain-free and subtle movement of the joint. Hy2Care’s hydrogel implant technology aims to facilitate the repair of traumatic cartilage defects with natively formed cartilage!
Hy2Care’s proprietary technology platform is based on the in-situ formation of the CartRevive® hydrogel implant. Two naturally derived polymers – Dextran and Hyaluronic acid conjugates– are mixed and injected into the defect. Once the mixture is applied to the cartilage defect, the components turn into a gel filling the defect in about a minute, through a mild enzymatic cross-linking reaction, while simultaneously attaching itself to the matrix proteins of the surrounding cartilage and bone. The gel forms a resorbable scaffold that degrades over time. Cells can subsequently grow into the scaffold and start depositing hyaline cartilage. In this way, the hydrogel aims to enable natural restoration of tissue defects by allowing cells from surrounding tissue to migrate into the matrix and start the natural recovery process towards the smooth hyaline cartilage.
The CartRevive® hydrogel implant can in principle be applied both through a minimally invasive open or an arthroscopic procedure. During our first clinical investigation a mini-open procedure will be performed.
.jpg)
The surgeon will initially prepare the defect through debridement, ensuring that the edges of the defect are smooth and consist of undamaged healthy cartilage. Subsequently the polymer mixture is applied through a specially designed dual chamber mixing syringe. The mixture is allowed to crosslink and form the hydrogel and to firmly attach to the surrounding cartilage and bone, which will take about a minute. Finally, the wound is closed.
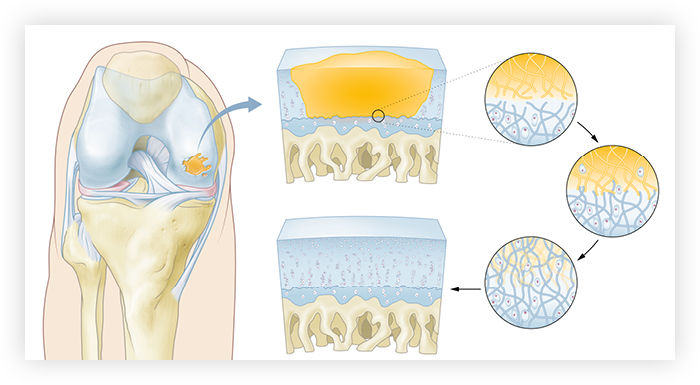
The open structure of the gel allows nutrients and chondrocytes (the human cells that occupy cartilage and are responsible for the formation of new cartilage) to enter and start the natural healing process. Over time the gel degrades and is replaced by naturally formed new tissue.
The CartRevive® hydrogel implant for cartilage repair is Hy2Care’s launching product and the first utilization of it’s Hydrogel technology platform. This platform allows for additional uses. Hy2Care® is also conducting early phase research on products for bone repair. Additionally, biologically active formulations of the gel are created aimed at pain relief and inflammation-reduction for patients in an advanced stage of OA.